Abstract
Introduction: Novel genetic classifications of diffuse large B-cell lymphoma (DLBCL) highlight the molecular complexity beyond cell of origin and provide new therapeutic implications. Next generation trials may incorporate novel agents for different genetic subtypes based on the pathogenesis mechanisms. For example, with the LymphGen classification, the MCD, BN2, ST2, and EZB subtypes are predicted to be susceptive to PI3K/mTOR targeting (Wright 2020). However, PI3K inhibitors have not been tested in the frontline, although data on the mTOR inhibitor everolimus were encouraging (Alliance 1085). We launched a phase 1/1b trial (NCT04323956) to investigate the feasibility of combining a novel PI3K inhibitor parsaclisib with standard R-CHOP immunochemotherapy and to seek an efficacy signal.
Methods: Adult patients with newly diagnosed DLBCL were eligible if any of the following was present: 1) non-GCB subtype per the Hans algorithm; 2) expression of either Myc (≥40%) or Bcl2 (≥50%) by immunohistochemistry; or 3) MYC rearrangement by FISH. High grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, regardless of morphology, was also eligible. All patients received parsaclisib plus R-CHOP. Phase 1 followed a 3+3 design and the primary endpoint was maximum tolerated dose (MTD) of parsaclisib. Dose levels tested were level 1 (20 mg QD, d1-10; starting level) and level 2 (20 mg QD, d1-14). MTD was defined as the dose level below the lowest dose that induced dose limiting toxicity (DLT) in at least one-third of patients. The primary endpoint in phase 1b is complete response (CR) rate by PET.
Results: From July 2020 to June 2021, 15 patients were enrolled, 9 in phase 1 and 6 in phase 1b. The median age at diagnosis was 56 years (range 20-79), and 7 (47%) were female. One patient (7%) had ECOG PS ≥2, 7 (47%) had elevated LDH, 7 (47%) had >1 extranodal site, 13 (87%) had stage III/IV, and 7 (47%) had high-intermediate or high risk International Prognostic Index. Pathology was DLBCL in 13 patients (2 with concurrent follicular lymphoma) and high grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements in 2 patients. Four (27%) had non-GCB subtype, 7 (47%) had Myc or Bcl2 single expression, 8 (53%) had Myc/Bcl2 double expression, and 5 (33%) had MYC rearrangement (2 with concurrent BCL2 and/or BCL6 rearrangements).
In phase 1, 3 patients were enrolled at dose level 1 and 6 patients were enrolled at dose level 2. No DLT was observed. Therefore, MTD was not reached, and dose level 2 was selected for phase 1b dose expansion. Six patients were enrolled in phase 1b to date.
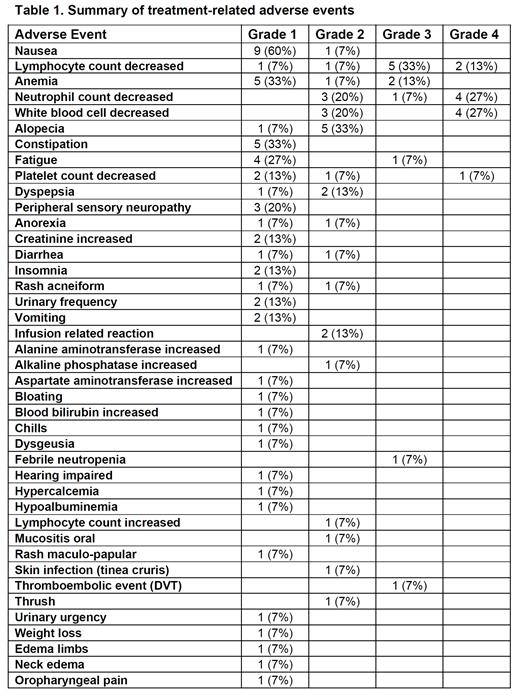
At the data cutoff date of 7/22/2021, 8 patients completed all 6 cycles of treatment, 1 competed 5 cycles, 3 completed 3 cycles, 2 completed 2 cycles, and 1 completed 1 cycle. Treatment-related adverse events (AE) are summarized in Table 1. The most common hematological AE included lymphopenia (60%), anemia (53%), neutropenia (53%), and thrombocytopenia (27%), and the most common non-hematological AE included nausea (67%), alopecia (40%), constipation (33%), fatigue (33%), dyspepsia (20%), and peripheral sensory neuropathy (20%). The most common grade 3 or 4 AE included lymphopenia (47%), neutropenia (33%), and anemia (13%). One 80-year-old female required parsaclisib dose reduction in cycle 3 and subsequent parsaclisib discontinuation as well as cyclophosphamide and doxorubicin dose reductions due to febrile neutropenia (no source of infection was identified). No other patients required dose reductions.
The median follow-up was 3.7 months (range 0.8-10.7). Thirteen patients were evaluable for interim response by PET. The objective response rate was 92%, with 8 (62%) CR and 4 (31%) partial response (PR). One (8%) patient progressed before cycle 2 following a transient clinical response (shrinking palpable mass). Among those who achieved an objective response at interim, 7 patients were evaluable for end of treatment response and all 7 maintained a response, with 6 (86%) CR and 1 (14%) PR.
Conclusions: Parsaclisib and R-CHOP combination therapy was generally well tolerated, with no DLT observed in phase 1 and no major safety concerns in both phase 1 and the ongoing phase 1b expansion. The preliminary efficacy signal of objective response appears encouraging in a small cohort of high risk patients. Parsaclisib plus R-CHOP can be an experimental arm for future frontline DLBCL trials investigating genetic subtype-driven novel therapies.
Wang: Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Genentech: Research Funding; MorphoSys: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. King: Celgene/BMS: Research Funding. Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Tun: Gossamer Bio, Acrotech: Consultancy; Mundipharma, Celgene, BMS, Acrotech, TG therapeutics, Curis, DTRM: Research Funding. Habermann: Seagen: Other: Data Monitoring Committee; Incyte: Other: Scientific Advisory Board; Tess Therapeutics: Other: Data Monitoring Committee; Morphosys: Other: Scientific Advisory Board; Loxo Oncology: Other: Scientific Advisory Board; Eli Lilly & Co.,: Other: Scientific Advisor. Witzig: Karyopharm Therapeutics, Celgene/BMS, Incyte, Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics: Research Funding. Nowakowski: Celgene, NanoString Technologies, MorphoSys: Research Funding; Celgene, MorphoSys, Genentech, Selvita, Debiopharm Group, Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Parsaclisib is an investigational agent used in this clinical trial.